Currently, COVID-19 is at the top of everyone’s minds. All of society is affected by the pandemic and hoping for a return to normalcy. However, it seems that the virus will not go away by itself. One of the most effective ways to end the pandemic is herd immunity. Herd immunity occurs when most of the population has the necessary antibodies to combat the virus. According to the Covid Symptom Study, herd immunity can be achieved by everyone getting infected, which is “extremely risky and costs thousands of lives”, or through vaccination, which is the safest method to provide immunity to people. Since the beginning of this pandemic, pharmaceutical companies have been working on the creation of an effective COVID-19 vaccine at an accelerated speed.
Most people have gotten vaccines in their lives, such as polio vaccines during childhood and the annual flu vaccines. A vaccine stimulates the immune system to develop the antibodies necessary for protection against the actual viruses so people don’t get sick. The vaccines given are significantly weakened or inactivated versions of the viruses, so they still trigger an immune reaction but are considered safe. Side effects may still occur due to an injection site reaction or an immune response to foreign particles. Once the body establishes an immune response, including antibodies, the human body will then have protections against that pathogen to keep people from getting infected. Some vaccines require multiple doses to act as “boosters” to the original dose, while others only require one.
Vaccines usually take years to develop, but under the current circumstances, the COVID-19 vaccine will be rushed. The mRNA platform that both Pfizer and Moderna are using, works by providing the genetic code from the SARS-CoV-2 virus’ spike proteins to trigger our immune reaction. This reaction produces the antibodies and T-cell responses needed to protect us from infection from the virus. mRNA can produce any protein, and it is much easier to manufacture and modify if there are mutations that occur in the current SARS-CoV-2 virus. This is especially important because a large amount of vaccines must be made and delivered to obtain herd immunity.
The genome of this virus was published in January 2020 and the development of the COVID-19 vaccine by Pfizer/BioNTech and Moderna began in March 2020. Clinical trials were conducted mainly in the US and Europe. Currently, phase 3 vaccine trials have been completed. Pfizer and Biotech announced their vaccine can “prevent 95% of cases of the disease”, while Moderna’s vaccine “has an estimated vaccine efficacy of 94.1%”. Both vaccine trials had no life-threatening adverse effects reported. The most common adverse effects are sore arms, muscle aches, and fevers. One of the greatest challenges for Pfizer is that the vaccine needs to be stored at -70 degrees Celsius, which makes distribution and storage harder. On the other hand, Moderna’s vaccine only requires storage at -20 degrees Celsius, the same temperature as a general freezer, making it easier to store. Operation Warp Speed has purchased 100 million vaccines from each Pfizer and Moderna, which can be deployed as soon as the FDA approves them.
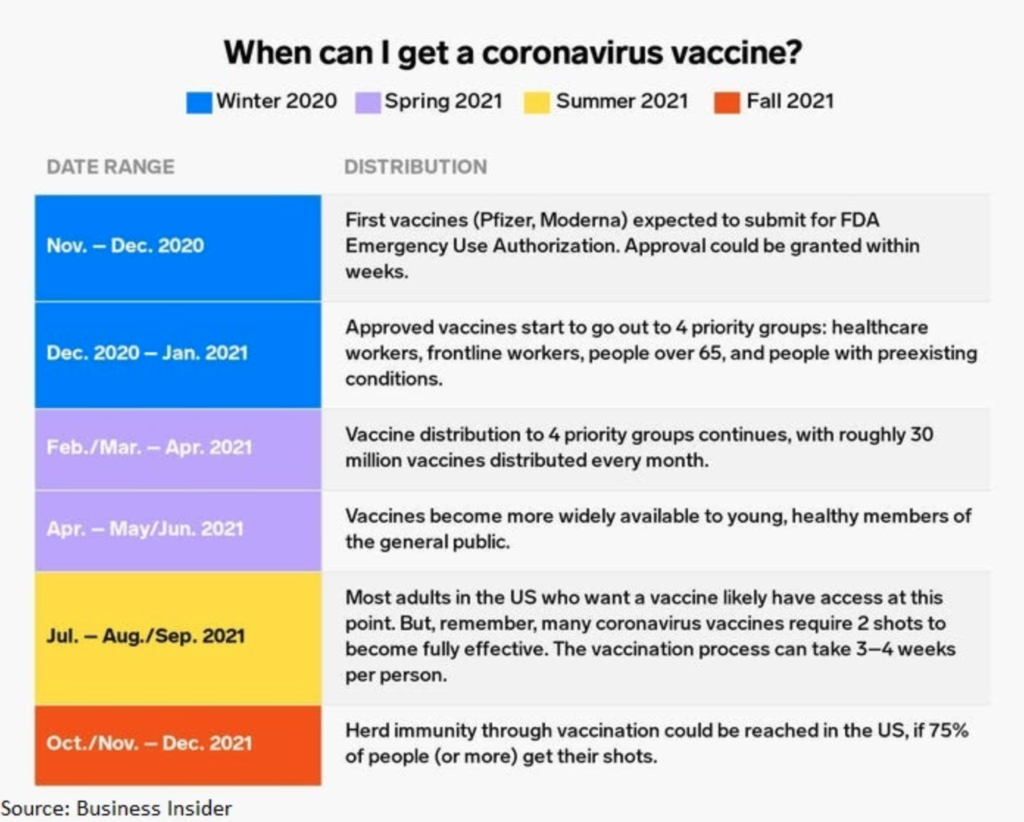
Assuming everything goes well, the first batch of vaccines will be available to first responders by the end of this year, and the public by mid 2021. Operation Warp Speed will get the vaccine doses out as soon as possible by vaccinating in phases, which is shown in the image below. It is encouraged that everyone gets a vaccine for the necessary herd immunity to take place so life can go back to normal.
Works Cited:
https://www.the-scientist.com/news-opinion/the-promise-of-mrna-vaccines-68202
https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
https://www.statnews.com/2020/11/18/pfizer-biontech-covid19-vaccine-fda-data/
https://www.pfizer.com/news/hot-topics/covid_19_vaccine_u_s_distribution_fact_sheet

